De-risking R&D of neurodegenerative drugs by versatile biomarker utility
Aggregated proteins are pathological hallmarks of CNS diseases. It is well-established that small oligomers and protofibrils act as the toxic drivers behind these disorders. Consequently, numerous drugs have been formulated to interfere with protein aggregation with the ultimate goal of curing the disease.
sFIDA, endowed with sub-femtomolar sensitivity, can quantify oligomers at the single-particle level. This makes it an invaluable tool in both pre-clinical and clinical applications. These applications include patient selection and stratification, therapy monitoring, and validation of Mechanism of Action (MoA) and target engagement.

How it works
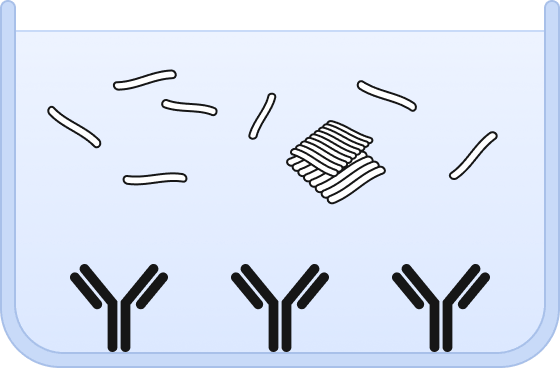
❶ Wells are chemically modified to bind a capture antibody and sample is added.
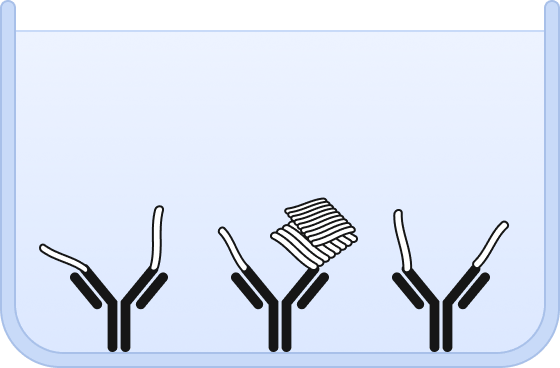
❷ Both, oligomers and monomers are bound by the capture antibody.
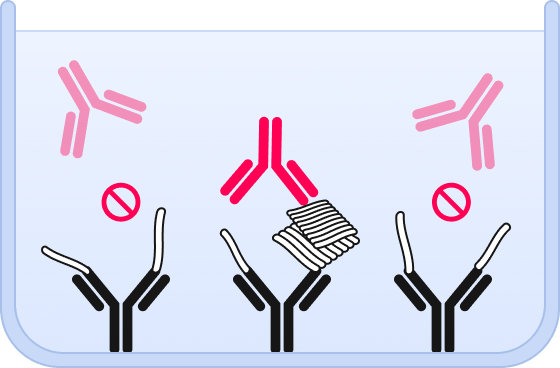
❸ Using the same epitope for capture and detection, only oligomers are probed – but no monomers.
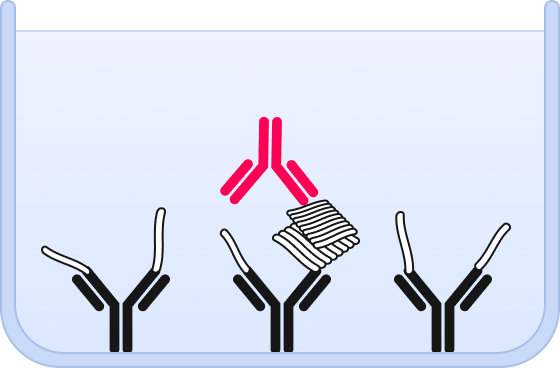
❹ Individual oligomers are detected and counted by highly sensitive fluorescence microscopy.
Aβ aggregate quantification in stool samples
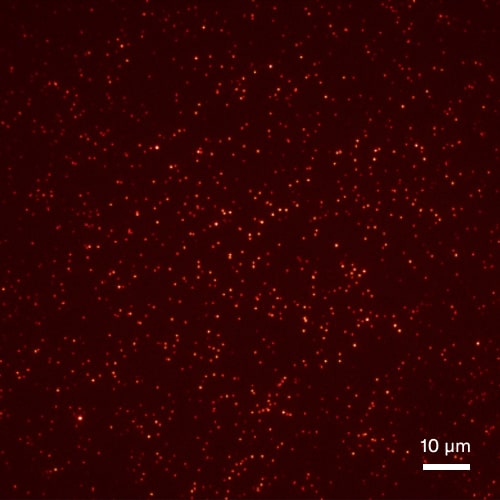
Synthetic Aβ aggregates
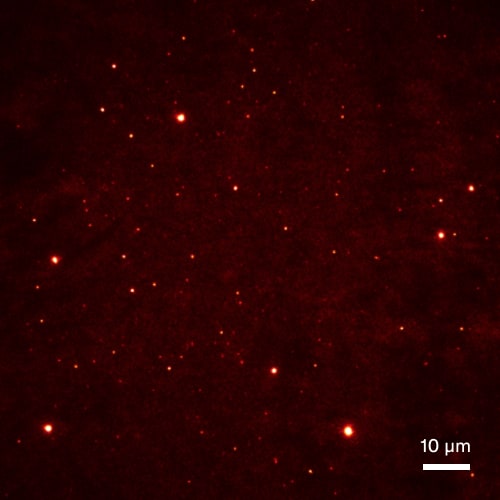
Aβ aggregates in AD stool samples
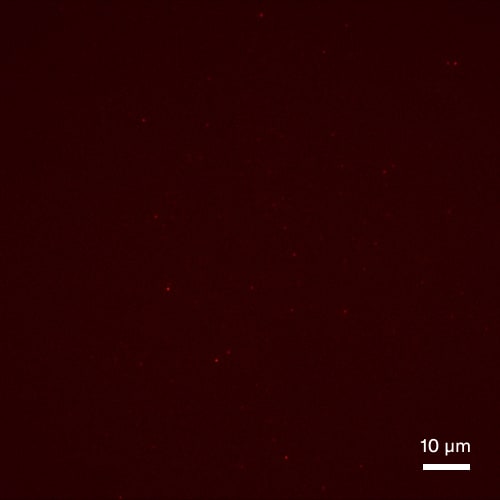
Buffer control