
Ultrasensitive quantitation and sizing of aggregates for development and QC of biologicals
Subvisible particles in biologics pose a significant challenge due to their potential to trigger toxic and immunogenic adverse effects.
attyloid’s sFIDA technology excels with its ultra-sensitive detection capabilities, identifying these particles in cell culture screening – an exceptionally upstream phase in the development process of biologicals.
This early-stage detection is crucial in mitigating risks and ensuring the robustness of your biologics R&D by pinpointing unstable clones before significant downstream resources are invested.
How it works
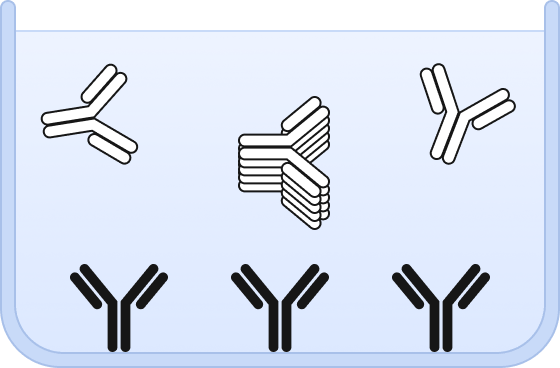
❶ Wells are chemically modified to bind a capture antibody and sample is added.
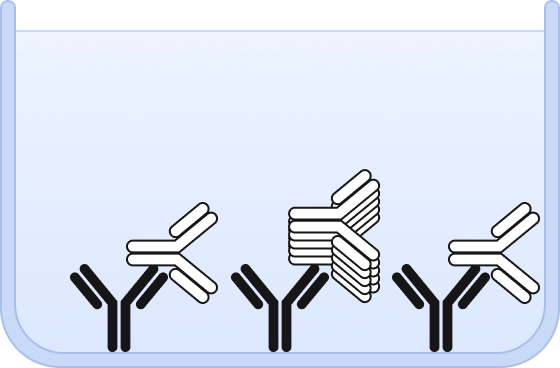
❷ Both, aggregates and monomers are bound by the capture antibody.
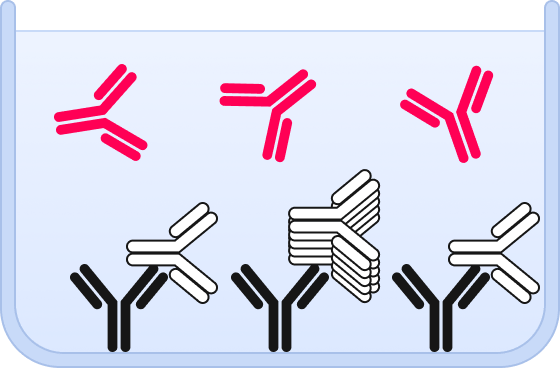
❸ A fluorescent detection probe is added that binds to the same epitope as the capture.
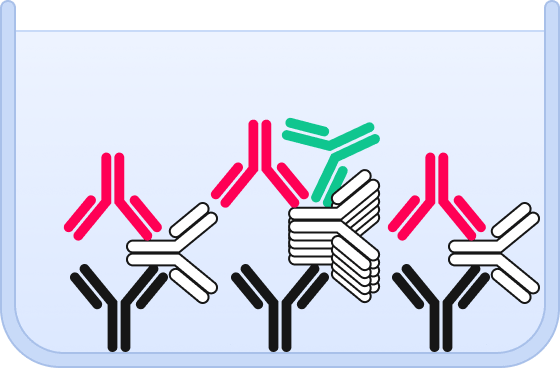
❹ By adding a second detection probe, aggregates can be specifically detected and quantified by dual color fluorescence microscopy.
Subvisible particle detection by sFIDA – seeing is believing.
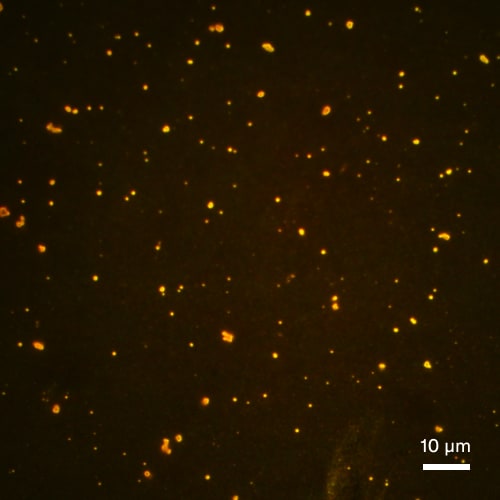
Stressed IgG sample
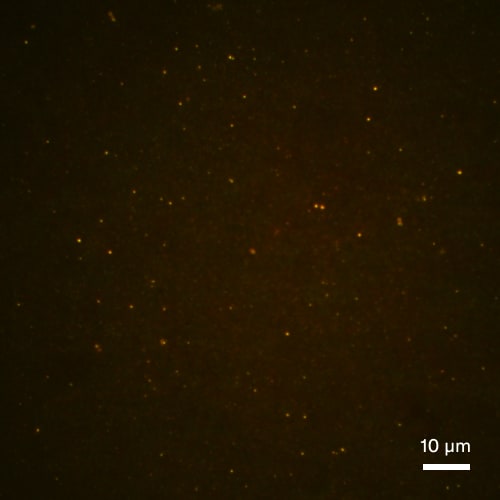
Supernatant after centrifugation at 100,000 g

Non-stressed control