Counting and characterization of viral particles in complex matrices
Accurately quantifying and characterizing viral particles is essential across a wide spectrum of biotechnological applications, such as Vaccine Production, Protein Expression, Viral Therapeutics, and Antiviral Development.
sFIDA technology is at the cutting edge of this scientific exploration, providing the capability to precisely ascertain virus and VLP titers, identify surface antigens with exceptional specificity, and differentiate between full and empty capsids. This facilitates a more refined and in-depth comprehension, fostering progress in both biotechnology and pharmaceutical fields.

How it works
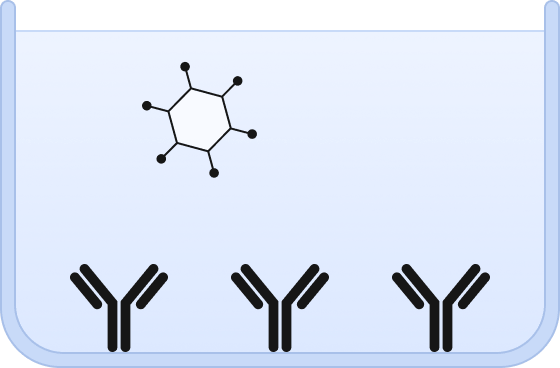
❶ Wells are chemically modified to bind a capture antibody and sample is added.
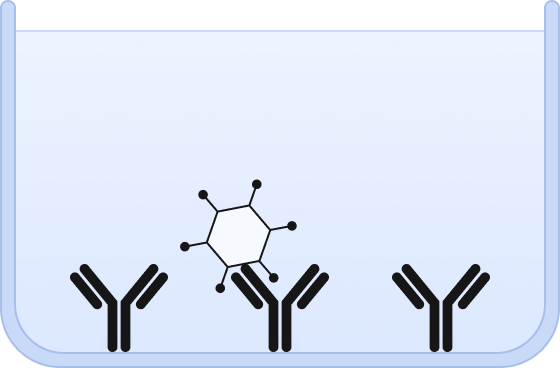
❷ Viral particles are immobilized on the capture surface.
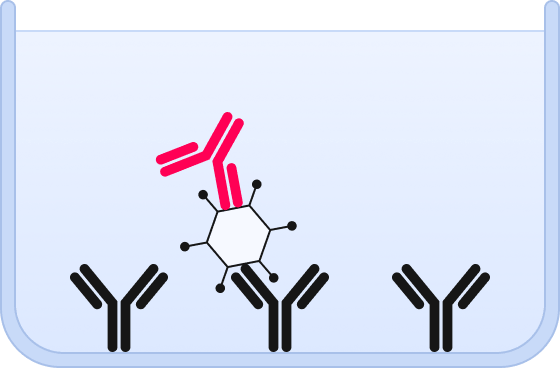
❸ A fluorescent probe directed against a capsid epitope is added.

❹ Optional: A second probe binds to a surface antigen.
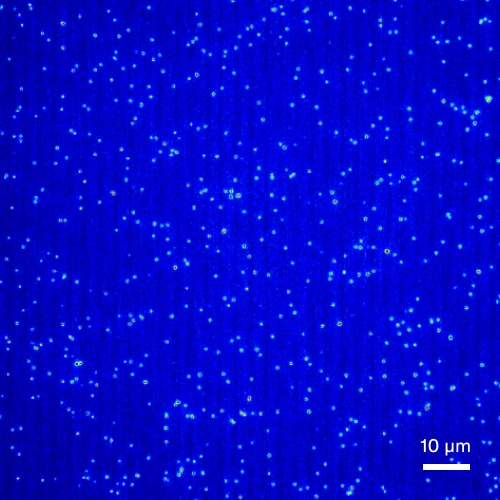
Quantitation and characterization of subresolution viral particles
AAV Log 10 Serial Dilution