our science
sFIDA is a platform technology for quantitation and sizing of single protein aggregates at unprecedented levels of sensitivity and specificity.
Surface-based Fluorescence Distribution Analysis
The sFIDA platform technology offers a cutting-edge approach for the quantitative detection of single oligomers and aggregates, which serve as biomarkers of central nervous system (CNS) diseases such as Alzheimer’s Disease (AD) and Parkinson’s Disease (PD). This platform integrates the precision of selective immunoassays with the superior sensitivity of fluorescence microscopy to count single particles. As a result, sFIDA can detect these biomarkers at unprecedented levels of sensitivity and specificity.
The versatility of the sFIDA platform is further highlighted by its successful application to a range of conditions and biological material, including brain homogenate, cerebrospinal fluid, blood plasma, and even stool samples. This broad applicability enables the use of sFIDA technology in diverse clinical and research settings, allowing for a more comprehensive understanding of the molecular basis of neurodegenerative disorders.
How it works
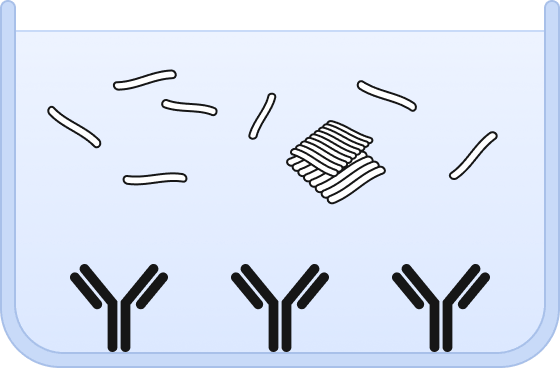
❶ Wells are chemically modified to bind a capture antibody and sample is added.
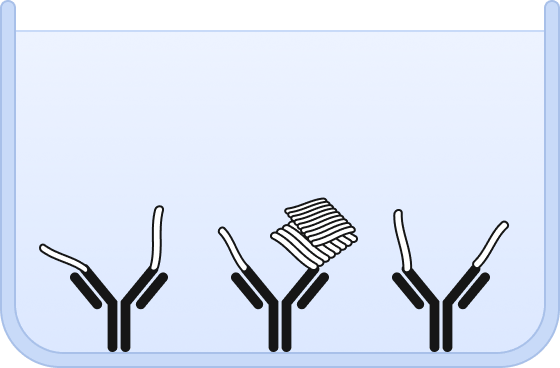
❷ Both, oligomers and monomers are bound by the capture antibody.
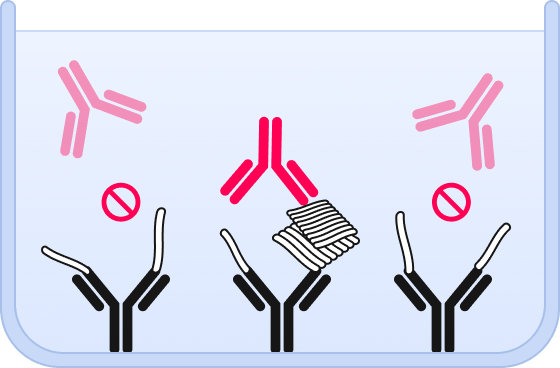
❸ Using the same epitope for capture and detection, only oligomers are probed – but no monomers.
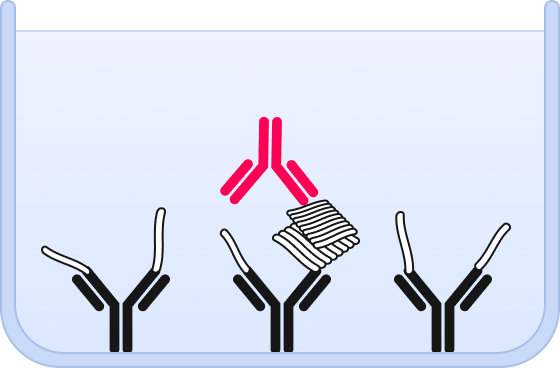
❹ Individual oligomers are detected and counted by highly sensitive fluorescence microscopy.
In addition, the sFIDA platform holds significant promise for advancing drug development. By providing valuable insights into the pathogenesis of CNS diseases and the molecular underpinnings of neurodegenerative disorders, sFIDA technology can aid in the identification of novel therapeutic targets and the evaluation of drug efficacy.
The sFIDA platform technology was developed at the Forschungszentrum Jülich, a leading research institute in Germany. Since 2018, the technology is commercialized by the spin-off company attyloid GmbH, which is dedicated to providing innovative solutions for the detection and analysis of biomarkers associated with neurodegenerative diseases.

attyloid is more than a biotech company.
We are an academicy spin-off dedicated to the scientific rigor that the complex fields of protein misfolding and CNS diseases demand. Our research plays a critical role in diagnosing and understanding these diseases and paving the way for innovative therapeutics.
Peer-reviewed publications
At attyloid, we value the importance of peer-reviewed research. Our work has been published in reputable scientific journals, giving us a solid footing in the field of protein misfolding and aggregation. Our proprietary sFIDA technology platform sets us apart. Its ultra-sensitive quantitative capabilities have been rigorously examined and validated through peer-reviewed research, ensuring unparalleled accuracy and reliability.